Overall Response Rate of 76% in Advanced Melanoma Patients with Dynavax’s SD-101 in Combination with KEYTRUDA® (pembrolizumab); Data Presented Today at the 2019 ASCO Annual Meeting
- Progression Free Survival (PFS) rate of 18-months in 72% of patients
- Clinical efficacy in patients with immunologically cold tumor at baseline
- The combination of SD-101 and pembrolizumab was well-tolerated, consistent with previous reports
BERKELEY, Calif.,
|
|||||
“To consistently see an overall response rate above 70% among advanced melanoma patients, which has historically been a difficult population to treat, is very encouraging and exciting,” said Robert Janssen, M.D., chief medical officer of Dynavax. “Additionally, in response to treatment, we have seen immunologically cold tumors reach similarly high levels of immune cell activation as immunologically hot tumors.”
The Phase 1b/2 clinical study (NCT02521870) in patients with advanced melanoma is ongoing. In the study, SD-101 is administered intratumorally with 8 mg in 1 lesion or 2 mg in 1–4 lesions combined with intravenous administration of 200 mg of pembrolizumab.
Key highlights from the clinical data presentation include:
- Efficacy:
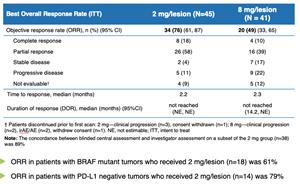
º The overall response rate (ORR) in the SD-101 2 mg/lesion group (76%) was higher than in the SD-101 8 mg/lesion group (49%)
º The median duration of response (DOR) in both groups has not been reached, with the lower bound of the 95% confidence interval of at least 14 months
º The 18-month progression free survival (PFS) rate in the SD-101 2 mg/lesion group (72%) was higher than in the SD-101 8 mg/lesion group (36%)
º Similar rates of responses occurred in patients with PD-L1 negative tumors and PD-L1 positive tumors
º Tumor shrinkage has been observed in both injected and non-injected lesions, including visceral lesions such as the liver and lung
- Immunologically cold tumors are a therapeutic challenge for anti-PD-1 therapy; the ability of SD-101 with pembrolizumab to convert cold tumors (PD-L1 negative, low IFNγ and T cell signature at baseline) into T cell rich tumors is demonstrated by biomarker data in the samples tested
º This ability to convert cold into inflamed tumors is consistent with similar effects in head and neck squamous cell carcinoma (HNSCC) (ASCO 2019, Abstract 6039)
- The superior induction of infiltrating effector immune cells in lesions treated with the 2 mg/lesion dose compared with 8 mg/lesion is consistent with the increased response observed
- The combination of SD-101 and pembrolizumab was well tolerated, consistent with previous report
º AEs associated with SD-101 were transient, mild to moderate injection-site reactions and flu-like symptoms that were manageable with over-the-counter medications
º No increase in immune-related AEs over pembrolizumab monotherapy were observed
A table accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d03e76d2-86e7-4675-ae1d-8a29251f5101
- The ORR in patients with BRAF mutant tumors who received 2 mg/lesion (n=18) was 61%
- The ORR in patients with PD-L1 negative tumors who received 2 mg/lesion (n=14) was 79%
In addition, the company presented data from the Phase 1b/2, open label, multicenter, study of the combination of SD-101 and pembrolizumab in patients with advanced/metastatic melanoma resistant to anti-PD-1/PD-L1 therapy.
Key highlights from the clinical data presentation include:
- Efficacy results from the combination of SD-101 and pembrolizumab in confirmed PD-1 resistant or refractory patients demonstrated an 19.4% ORR in the SD-101 2 mg/lesion group and a 13.3% ORR in the SD-101 8 mg/lesion group
- Responses were observed in SD-101 injected and non-injected lesions (including liver and lung metastases)
- Responses and disease control were observed in BRAF mutant or wild type tumors
- Biomarker data demonstrate that SD-101 added to pembrolizumab significantly changes the tumor microenvironment of patients that previously failed PD-1 blockade including the infiltration of activated T cells, NK cells, and B cells
- The combination of SD-101 and pembrolizumab was well tolerated, consistent with previous reports
º No evidence of an increased incidence or severity of AEs over pembrolizumab monotherapy
º No increase in immune-related AEs over pembrolizumab monotherapy
º AEs associated with SD-101 were mainly mild to moderate injection-site reactions and flu-like symptoms that were manageable with over-the-counter medications
In addition, a poster titled: “Overcoming genetically based resistance mechanisms to PD-1 blockade” (Abstract No: 2584) was presented. The goal of the preclinical study was to assess mechanism-based strategies to overcome resistance to anti-PD1 therapy. Results demonstrated that even in the extreme setting of genetic resistance to PD-1 blockade by JAK1/2 LoF, resistance can be overcome by SD-101, a TLR9 agonist.
About SYNERGY-001 (KEYNOTE-184)
SYNERGY-001, previously referred to as MEL-01, is a Phase 1b/2 SYNERGY-001/KEYNOTE-184 trial in combination with KEYTRUDA which includes patients with histologically or cytologically confirmed unresectable Stage IIIC/IV melanoma. The primary endpoint of the trial is objective response rate assessed by RECIST v1.1. The secondary endpoints are safety and tolerability, progression-free survival and duration of response, with an exploratory endpoint of immunophenotype of the tumor microenvironment.
About SD-101
SD-101 is a proprietary, second-generation, Toll-like receptor 9 (TLR9) agonist CpG-C class oligodeoxynucleotide. Dynavax is evaluating SD-101 in several clinical studies to assess its safety and activity, including a Phase 2 study in combination with KEYTRUDA® (pembrolizumab) in advanced melanoma and metastatic or recurrent head and neck squamous cell cancer in collaboration with Merck, and in high risk breast cancer in collaboration with I-SPY 2. Dynavax maintains all commercial rights to SD-101.
About Dynavax
Dynavax is a fully-integrated biopharmaceutical company focused on leveraging the power of the body's innate and adaptive immune responses through toll-like receptor (TLR) stimulation. Dynavax discovers and develops novel vaccines and immuno-oncology therapeutics. The Company is currently exploring strategic alternatives for its immuno-oncology portfolio. The Company launched its first commercial product, HEPLISAV-B® [Hepatitis B Vaccine (Recombinant), Adjuvanted], in
Forward-Looking Statements (update)
This press release contains "forward-looking" statements, including statements regarding the conduct of clinical trials of SD-101, including results from the Phase 1b/2 trial, and potential value of SD-101 across multiple tumor types. Actual results may differ materially from those set forth in this press release due to the risks and uncertainties inherent in our business, including whether we can timely provide adequate clinical supplies; initiation, enrollment and completion of clinical trials of SD-101; whether interim and final results of current and future clinical trials will support the initiation or continuation of subsequent trials by us or another party; issues arising in the regulatory process; the ability to successfully develop or pursue strategic alternatives for SD-101, including the funding of future studies as well as other risks detailed in the "Risk Factors" section of our Annual Report on Form 10-K for the fiscal year ended
Contact: Heather Rowe Vice President, Investor Relations& Corporate Communications hrowe@dynavax.com 510-665-7269
Source: Dynavax Technologies Corporation